

PREPARE TO PROVIDE YOUR RECOMMENDATION.
TAKE THE INFLUENZA VACCINE CHALLENGE.
Before administration, please see the Prescribing Information for Flublok® Quadrivalent.
This activity is sponsored by VaxServe®, Inc.
Welcome to the INFLUENZA VACCINE CHALLENGE. As a pharmacist, you can help identify the immunization needs of adults 50 years and older coming into the pharmacy and provide the appropriate influenza vaccine recommendation.
Flublok Quadrivalent is a vaccine indicated for active immunization against disease caused by influenza A subtype viruses and influenza type B viruses contained in the vaccine. Flublok Quadrivalent is approved for use in persons 18 years of age and older.
Your task is to watch an informational video and answer 6 questions about Flublok® Quadrivalent, an option that can be considered when an older adult comes into the pharmacy for their influenza vaccination.1,2
Answer all 6 questions correctly and print a personalized certificate recognizing your knowledge regarding Flublok® Quadrivalent and your preparedness to counsel on this option.
Watch the
VIDEO
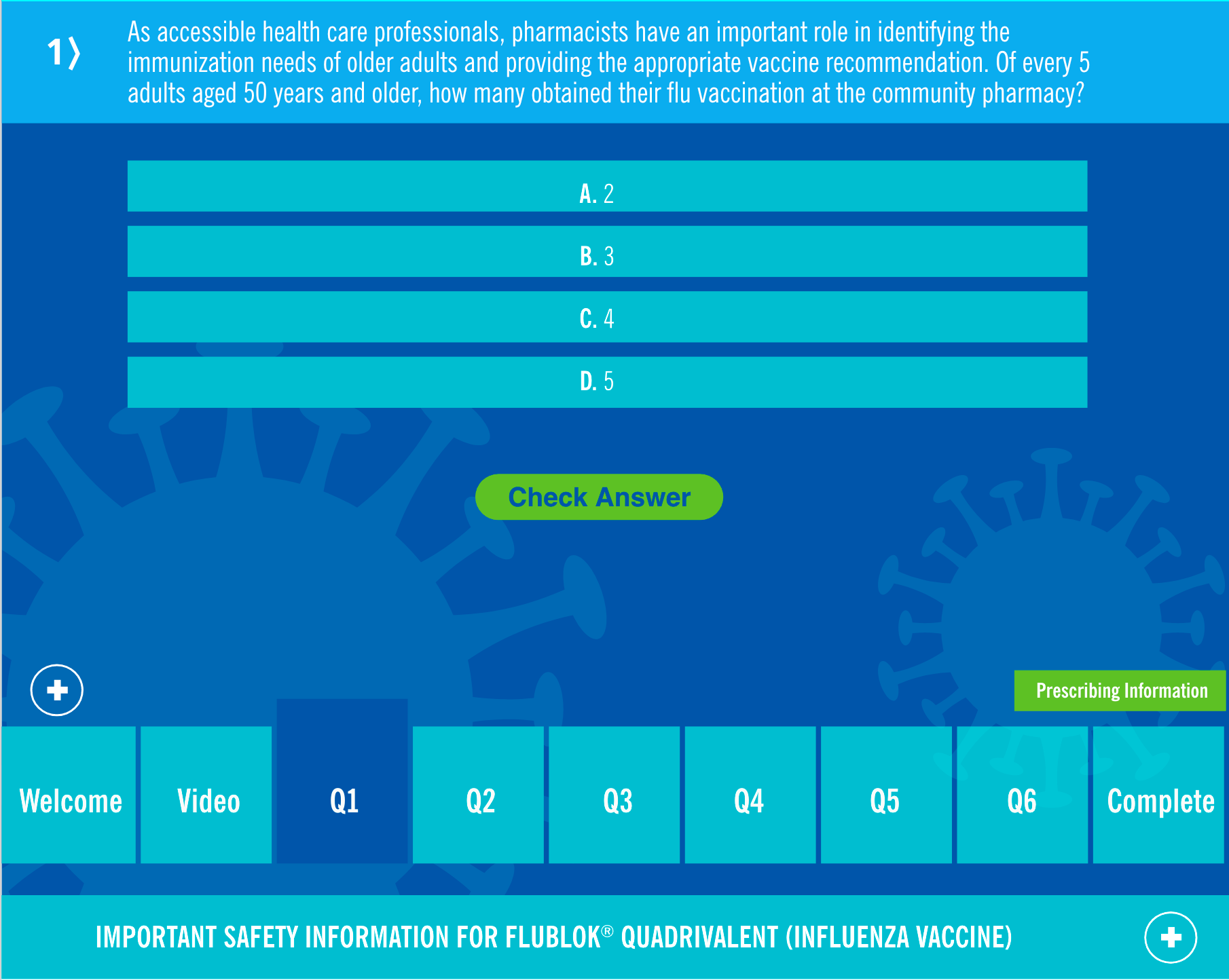
Answer the
QUESTIONS
Complete the
CHALLENGE






IMPORTANT SAFETY INFORMATION FOR FLUBLOK® QUADRIVALENT (INFLUENZA VACCINE)
Flublok Quadrivalent should not be administered to anyone who has had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
If Guillain-Barré syndrome has occurred within 6 weeks following previous influenza vaccination, the decision to give Flublok Quadrivalent should be based on careful consideration of the potential benefits and risks.
IMPORTANT SAFETY INFORMATION FOR FLUBLOK® QUADRIVALENT (INFLUENZA VACCINE)
Flublok Quadrivalent should not be administered to anyone who has had a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine.
If Guillain-Barré syndrome has occurred within 6 weeks following previous influenza vaccination, the decision to give Flublok Quadrivalent should be based on careful consideration of the potential benefits and risks.
If Flublok Quadrivalent is administered to immunocompromised persons, including those receiving immunosuppressive therapy, the immune response may be lower than expected.
Vaccination with Flublok Quadrivalent may not protect all recipients.
For Flublok Quadrivalent, in adults 18 through 49 years of age, the most common injection-site reactions were tenderness and pain; the most common solicited systemic adverse reactions were headache, fatigue, myalgia, and arthralgia. In adults 50 years of age and older, the most common injection-site reactions were tenderness and pain; the most common solicited systemic adverse reactions were headache, and fatigue. Other adverse reactions may occur.
Before administration, please see the Prescribing Information for Flublok Quadrivalent.
MAT-US-2105501-v1.0-06/2022
© 2022 MJH Life Sciences™ and Pharmacy Times. All rights reserved.
06/2022 VAX8313